Service and Support for Registration With the Chinese National Medical Products Administration (NMPA) and Others
- Service and support for registration of pharmaceutical products, pharmaceutical packing materials, health foods, and medical devices with China NMPA
- Support for preparation of Investigator's Brochure and protocols, negotiations with clinical trial sites, and arrangement for contract research agreement
- Obtaining China Compulsory Certificate (CCC) required for products belonging to the 7 categories such as an artificial heart-lung machine
Practical Market Research
- Interviews with medical specialists, brainstorming with clinicians
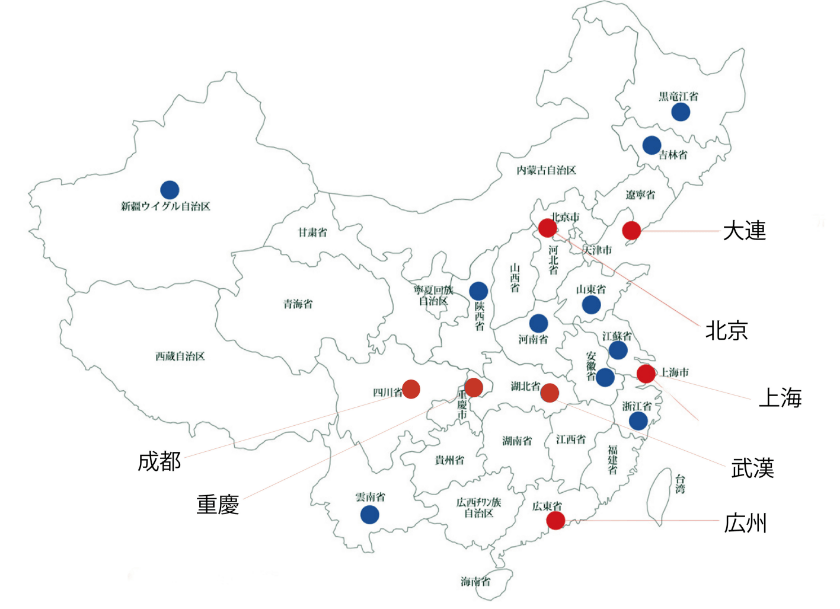
Face-to-face survey with clinicians of major medical schools and university hospitals in locations such as Beijing, Shanghai, and Guangzhou
Example: 30 questions regarding markets and new products
Facilities: Beijing Hospital, Beijing Tongren Hospital, university hospitals (Dalian, Tianjin, etc.), People's Hospitals (Dalian, Jiangsu, Zhongshan, etc.) - Pricing, sales promotion, development of distributor's policies suitable for the market through the consulting physician of our company
- Other services such as support for clinical trial sites, academic reports and papers, and arrangement for publication
- Test marketing and monitoring survey in designated areas and facilities
- Sales promotion, assistance for Japanese medical representatives, sales attendant
- Management and operation of facilities such as plants, export and import operations
- Support for product development in China
- Integrated support until startups of plants and offices
1. What is China NPMA Certification?
- China NMPA formulates requirements to be satisfied in order to sell medical devices, pharmaceutical products, pharmaceutical packaging materials, and health foods in the Chinese market.
- This certificate is equivalent to Manufacturing/Marketing Approval Application established under the jurisdiction of the Ministry of Health, Labour and Welfare in Japan.
(conforming to PMA certification of FDA in the US and CE certification in Europe)
Registration With and Notification to China NMPA
- Whether the product needs registration or notification varies depending on the category of the product to be applied for NMPA certification.
Classification:
For medical device: First-class (notification), second-class, third-class (registration)
For health food: 22 types of vitamins and minerals specified as nutritional supplements) (notification), 27 health functional foods (registration)
For pharmaceutical product: All chemical drugs, biological drugs, botanical drugs, new drugs, and generic drugs need registration
- Reregistration system: Registration certificate has an expiration date
(Four or five years depending on the item. Registration must be updated before the expiration date to maintain or continue registration.)
Registration must be updated before the expiration date to maintain or continue registration - Other: For procedures such as application for changes of medical devices, please contact us.
- Reregistration system: Registration certificate has an expiration date
- Requirements for products to be registered with China NMPA
A product launched in the market of the country or region where the applicant or the manufacturing facility is located. - Requirements for applicants of registration with China NMPA
A person who has been authorized to sell the product in the country of origin, or in the country or region where the manufacturing facility is located. - Requirements for relevant organizations and facilities necessary for the application for registration
Organizations that must be established prior to registration when the applicant does not have related parties in China.- Consigned agency in China (designated agent in China)
- Sales service agency
2. Time Period Required for Registration
- Although the time period required for the registration of medical devices varies, the following standard shall apply:
- First-class (notification): Registration is considered to have been approved when the officer in charge of receipt of the application receives the documents prepared and submitted to NMPA
- Second-class and Third-class: One year or more (it is difficult to predict the time period depending on whether or not the product has been exempted from clinical trials in China.)
Note:
First-class: The registration period is determined by the period of preparation for documents before the receipt.
For the second-class and third-class, type tests and clinical trials must be performed in China.
A product may be exempt from clinical trials in China in the following cases:- If the product is on the list of items that can be exempted from clinical trials, which is promulgated by NMPA.
- If the exemption is approved by NMPA after screening the evaluation report on the clinical trials of the same type of product that has already been certified.
- For health foods, pharmaceutical products, and pharmaceutical packaging materials: two and a half years or more
- The time period required for NMPA registration depends on the accuracy and completeness of the documents/data possessed by the applicant.
However, the period may be shortened through close communication and cooperation between the applicant and the agent for the application.
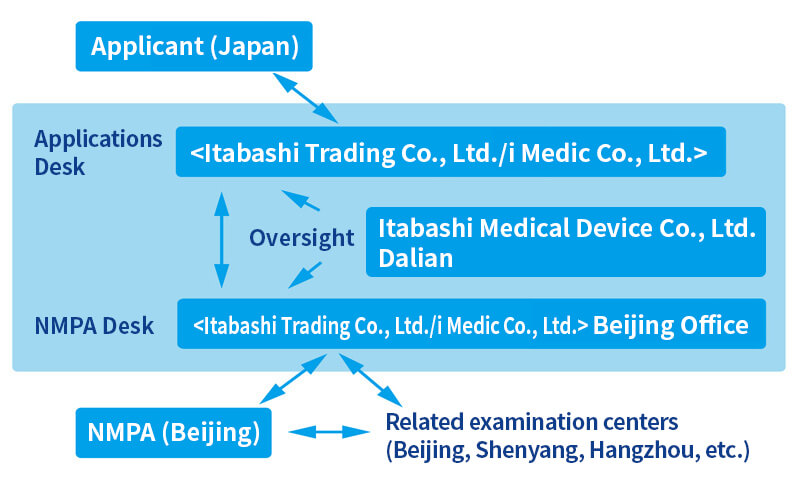
Please refer to Our Service System for the tripolar system operated by the Itabashi Group in its Tokyo, Dalian, and Beijing offices.
3. Expenses Required for Registration
- After specific information on the product outline is given, we will provide you with a formal quotation for each item as described below.
- Registration service fee (including all expenses except for inspection test of product samples, translation fee, and notarization fee)
- Product inspection test fee (for reference only)
- Application fee to be paid to NMPA (required tentatively for the application for medical devices and pharmaceutical products)
Note:
With regard to A., there are no additional fees to any situation that may arise during the process between the start of application and completion of registration, including negotiations with NMPA, test condition management, and various coordination processes. (within the initial budget)
B. As for the product inspection test fee, in principle, the actual cost will be billed to the customer at a later date with the invoice issued by the NMPA-certified testing laboratory. However, you may be required to pay an estimated amount of the fee as a deposit prior to the test. At the initial stage, we will give you an estimate for your reference. (to grasp the overall budget) -
We can give you an estimate based on a simple product description. Please feel free to contact us by phone or email.
Phone: +81-3-3248-1006
Email: - Our company will handle the product information of the applicant with care upon signing the confidentiality agreement, together with our subsidiaries in China (Dalian Head Office, Beijing Office).
4. Our Service System
- Our company group has established a system that provides attentive services and support from the two aspects of Japan side and China side (Dalian, Beijing), striving to accelerate the registration process through close communication with the related parties.
- Japan: Communication in Japanese (avoiding misunderstanding and misinterpretation)
- China: Close contact with NMPA at the local sites